其他細胞培養試劑
bFGF-2 (146 aa)

RCHEOT002 (25ug) RCHEOT003 (250ug) 1 mg/mL solution
Human Recombinant FGF-2 (146 aa) (bFGF)
鹼性纖維母細胞生長因子
bFGF 是人類胚胎幹細胞和 iPS 細胞培養基的關鍵成份,ReproCell 重組人類 FGF-2 (bFGF) 的 146 個氨基酸,使細不管在餵養細胞有或無的培養情況下,皆能維持多能性幹細胞的正常生長。bFGF 使用的濃度為 4-5 ng/ml,於 – 80℃可長期保存。
餵養細胞系統中所需的塗佈溶液
ReproCoat™
餵養系統培養靈長類幹細胞與 iPS 細胞之塗佈溶液
全球有許多實驗室使用,無菌可直接使用,常溫保存。適合許多種幹細胞培養。一瓶可塗佈 150 盤 6cm 培養皿。
細胞剝離液
Dissociation solution for human ES/iPS cells
靈長類幹細胞與 iPS 細胞用剝離液
剝離液是專門為靈長類胚胎幹細胞和 iPS 細胞所設計,可使細胞溫和且高效率剝離,無須刮取細胞,提高繼代後的細胞存活率 (較 Trypsin 好十倍)。適用於自動化細胞培養操作程序。剝離液開封後即可使用且不含血清,保持細胞一致的活性,減少細胞代數間的變異性。數代培養後,染色體核型維持正常。

冷凍保存液
Freezing Medium for Human ES/iPS cells
靈長類幹細胞與 iPS 細胞用冷凍保存液
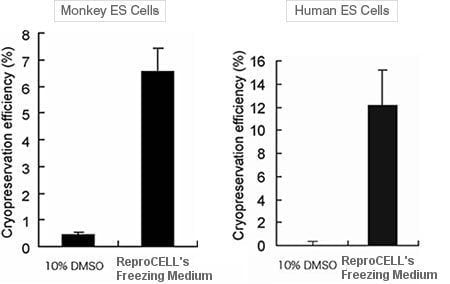
冷凍保存幹細胞是攸關能否得到快速及可靠結果的關鍵步驟。ReproCell 的冷凍保存液是專門對靈長類的胚胎幹細胞及 iPS 細胞所研發,確保胚幹細胞及 iPS 細胞解凍後的存活率,其效果比 DMSO 好十倍以上且可以立即使用。
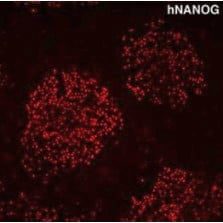
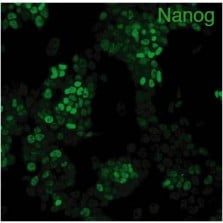
人類 / 老鼠 Nanog 多株抗體
Anti Nanog antibody
抗 Nanog 抗體
自 2004 年起全球銷售,抗體純度高,可清楚辨識 幹細胞表面特殊蛋白 Nanog。
bFGF
請左右移動表格位置查看更完整內容
| 產品貨號 | 產品名稱 | 產品容量 |
|---|---|---|
| RCHEOT002 | bFGF | 25ug |
| RCHEOT003 | bFGF | 250ug |
餵養細胞系統中所需的塗佈溶液
請左右移動表格位置查看更完整內容
| 產品貨號 | 產品名稱 | 產品容量 |
|---|---|---|
| RCHEOT001 | ReproCoat | 500mL |
細胞剝離液
請左右移動表格位置查看更完整內容
| 產品貨號 | 產品名稱 | 產品容量 |
|---|---|---|
| RCHETP002 | Dissociation solution for human ES/iPS cells | 30mL |
冷凍保存液
請左右移動表格位置查看更完整內容
| 產品貨號 | 產品名稱 | 產品容量 |
|---|---|---|
| RCHEFM001 | Freezing medium for human ES/iPS cells | 25mL |
人類 / 老鼠 Nanog 多株抗體
請左右移動表格位置查看更完整內容
| 產品貨號 | 產品名稱 | 產品容量 |
|---|---|---|
| RCAB001P | Anti mouse Nanog antibody | 200μL |
| RCAB002P-F | Anti mouse Nanog antibody | 100μL |
| RCAB003P | Anti human Nanog Antibody | 200μL |
| RCAB004P-F | Anti human Nanog Antibody | 100μL |
bFGF
出版物
- Hirata, Nao, et al. “A Chemical Probe that Labels Human Pluripotent Stem Cells.” Cell reports 6.6 (2014): 1165-1174.
- Isono, Kaori, et al. “Generation of familial amyloidotic polyneuropathy-specific induced pluripotent stem cells.” Stem Cell Research (2014).
- Luo, Lan, et al. “Effects of antioxidants on the quality and genomic stability of induced pluripotent stem cells.” Scientific reports 4 (2014).
- Mull, Amber N., Amanda Klar, and Christopher S. Navara. “Differential Localization and High Expression of SURVIVIN Splice Variants in Human Embryonic Stem Cells but not in Differentiated Cells Implicate a Role for SURVIVIN in Pluripotency.” Stem Cell Research (2014).
- Fukamachi, Hiroshi, et al. “CD49fhigh Cells Retain Sphere-Forming and Tumor-Initiating Activities in Human Gastric Tumors.” PloS one 8.8 (2013): e72438.
- Haraguchi, Yuji, et al. “Simple suspension culture system of human iPS cells maintaining their pluripotency for cardiac cell sheet engineering.” Journal of tissue engineering and regenerative medicine (2013).
- Liu, Yang, Shinji Sakai, and Masahito Taya. “Impact of the composition of alginate and gelatin derivatives in bioconjugated hydrogels on the fabrication of cell sheets and spherical tissues with living cell sheaths.” Acta biomaterialia 9.5 (2013): 6616-6623.
- Murakami, Masashi, et al. “The use of granulocyte-colony stimulating factor induced mobilization for isolation of dental pulp stem cells with high regenerative potential.” Biomaterials 34.36 (2013): 9036-9047.
- Nakajima-Takagi, Yaeko, et al. “Role of SOX17 in hematopoietic development from human embryonic stem cells.” Blood 121.3 (2013): 447-458.
- Takase, Osamu, et al. “The Role of NF-κB Signaling in the Maintenance of Pluripotency of Human Induced Pluripotent Stem Cells.” PloS one 8.2 (2013): e56399.
- Terai, Hideki, et al. “Activation of the FGF2-FGFR1 Autocrine Pathway: A Novel Mechanism of Acquired Resistance to Gefitinib in NSCLC.” Molecular Cancer Research 11.7 (2013): 759-767.
- Higuchi, Takuma, et al. “High Expression of Nuclear Factor 90 (NF90) Leads to Mitochondrial Degradation in Skeletal and Cardiac Muscles.” PloS one 7.8 (2012): e43340.
- Koyama, Noriaki, et al. “Human induced pluripotent stem cells differentiated into chondrogenic lineage via generation of mesenchymal progenitor cells.” Stem cells and development 22.1 (2012): 102-113.
- Ishiwata, Toshiyuki, et al. “Enhanced expression of fibroblast growth factor receptor 2 IIIc promotes human pancreatic cancer cell proliferation.” The American journal of pathology 180.5 (2012): 1928-1941.
- Kunisada, Yuya, et al. “Small molecules induce efficient differentiation into insulin-producing cells from human induced pluripotent stem cells.” Stem cell research 8.2 (2012): 274-284.
- Matsuura, Katsuhisa, et al. “Creation of human cardiac cell sheets using pluripotent stem cells.” Biochemical and biophysical research communications 425.2 (2012): 321-327.
- Nomura, Yoshiaki, et al. “Human periodontal ligament fibroblasts are the optimal cell source for induced pluripotent stem cells.” Histochemistry and cell biology 137.6 (2012): 719-732.
- Sato, Atsuki, et al. “Expression and role of nestin in human cervical intraepithelial neoplasia and cervical cancer.” International journal of oncology 41.2 (2012): 441-448.
- Shimojima, Keiko, et al. “Reduced PLP1 expression in induced pluripotent stem cells derived from a Pelizaeus?Merzbacher disease patient with a partial PLP1 duplication.” Journal of human genetics 57.9 (2012): 580-586.
- Yabe, Tomio, et al. “A peptide found by phage display discriminates a specific structure of a trisaccharide in heparin.” Journal of Biological Chemistry 286.14 (2011): 12397-12406.
- YAMAMOTO, TeTSuSHI, TOSHIYuKI ISHIwATA, and ZENYA NAITO. “Morphological and cytoskeletal alterations of nervous system tumor cells with different culturing methods.” International journal of oncology 38 (2011): 1253-1258.
Dissociation solution for human ES/iPS cells
出版物
- Tsuchida N; Kojima J; Fukuda A; Oda M; Kawasaki T; Ito H; Kuji N; Isaka K; Nishi H; Umezawa A; Akutsu H. Transcriptomic features of trophoblast lineage cells derived from human induced pluripotent stem cells treated with BMP 4. Placenta in press :1053 (2019).
- Kuroda T; Yasuda S; Tachi S; Matsuyama S; Kusakawa S; Tano K; Miura T; Matsuyama A; Soto Y. SALL3 expression balance underlies lineage biases in human induced pluripotent stem cell differentiation. Nature Commun 10:2175 (2019).
- Thiel A; Yavanian G; Nastke M-D; Morales P; Kouris NA; Kimbrel EA; Lanza R. Human embryonic stem cell-derived mesenchymal cells preserve kidney function and extend lifespan in NZB/W F1 mouse model of lupus nephritis. Sci Rep 5:17685 doi:10.1038/srep17685 (2015)
- Morizane R, et al. “Nephron organoids derived from human pluripotent stem cells model kidney development and injury.” Nat Biotechnol. 2015 Oct 12. doi: 10.1038/nbt.3392.
- Kumazaki, Tsutomu, et al. “Re‐emergence of undifferentiated cells from transplants of human induced pluripotent stem cells as a possible risk factor of tumourigenesis.” Cell Biology International Reports (2014).
- Delacote, Fabien, et al. “High frequency targeted mutagenesis using engineered endonucleases and DNA-end processing enzymes.” PloS one 8.1 (2013): e53217.
- Miyazaki, Takamichi, Norio Nakatsuji, and Hirofumi Suemori. “Optimization of slow cooling cryopreservation for human pluripotent stem cells.” genesis (2013).
- Razak, Siti Razila Abdul, et al. “Profiling of MicroRNA in Human and Mouse ES and iPS Cells Reveals Overlapping but Distinct MicroRNA Expression Patterns.” PloS one 8.9 (2013): e73532.
- Kuroda, Takuya, et al. “Highly sensitive in vitro methods for detection of residual undifferentiated cells in retinal pigment epithelial cells derived from human iPS cells.” PloS one 7.5 (2012): e37342.
- Kobayashi, Hideyuki. “Pluripotent Stem Cells Induced from Testicular Tissue of a Man with Klinefelter Syndrome (47, XXY) by Four Transcription Factors (OCT4, SOX2, KLF4, and C-MYC).” (2011).
- Kumazaki, Tsutomu, et al. “Establishment of human induced pluripotent stem cell lines from normal fibroblast TIG-1.” Human cell 24.2 (2011): 96-103.
- Shirasawa, Sakiko, et al. “Pancreatic exocrine enzyme-producing cell differentiation via embryoid bodies from human embryonic stem cells.” Biochemical and biophysical research communications 410.3 (2011): 608-613.
- Oka, Y., et al. “293FT cells transduced with four transcription factors (OCT4, SOX2, NANOG, and LIN28) generate aberrant ES-like cells.” J Stem cell Regenerative Med 3 (2010): 149-56.
- Lee, Tae-Hee, et al. “Functional recapitulation of smooth muscle cells via induced pluripotent stem cells from human aortic smooth muscle cells.”Circulation research 106.1 (2010): 120-128.
- Shimoji, Kenichiro, et al. “G-CSF promotes the proliferation of developing cardiomyocytes in vivo and in derivation from ESCs and iPSCs.” Cell Stem Cell6.3 (2010): 227-237.
- Shimoji, Kenichiro, et al. “G-CSF promotes the proliferation of developing cardiomyocytes in vivo and in derivation from ESCs and iPSCs.” Cell Stem Cell6.3 (2010): 227-237.
- Yoshie, Susumu, et al. “Bone morphogenetic protein-4 promotes induction of.” J Cell Sci 122.17 (2009): 3070-3082.
Freezing Medium for human ES and iPS Cells
出版物
- Saeki, Kumiko. “Feeder-Free Culture for High Efficiency Production of Subculturable Vascular Endothelial Cells from Human Embryonic Stem Cells.” Human Embryonic and Induced Pluripotent Stem Cells. Humana Press, (2012):77-294.
- Toyoda, Masashi, et al. “Generation of Induced Pluripotent Stem Cells from Human Amnion Cells.” Human Embryonic and Induced Pluripotent Stem Cells. Humana Press, (2012): 249-264.
- Akasaka, Tsukasa, et al. “Maintenance of hemiround colonies and undifferentiated state of mouse induced pluripotent stem cells on carbon nanotube-coated dishes.” Carbon 49.7 (2011): 2287-2299.
- Kumazaki, Tsutomu, et al. “Establishment of human induced pluripotent stem cell lines from normal fibroblast TIG-1.” Human cell 24.2 (2011): 96-103.

