以日本黃金標準技術製備培養液
通過嚴格的質量審查維持一致的品質
使用流程
請左右移動表格位置查看更完整內容
| 培養基 Culture Medium | 塗佈液 Coating Solution | 解離液 Dissociation Solution | 凍存液 Freezing Medium | 多能性生物標誌 Pluripotency Markers | ||
|---|---|---|---|---|---|---|
| 餵養細胞系統 (Feeder-dependent) | Primate ES Cell Medium | FGF-2 (146aa) | ReproCoat | Dissociation Solution | Freezing Medium | Anti Nanog antibody |
| 無餵養細胞系統 (Feeder-free) | ReproFF2 | Laminin-5 (Lanminin-332) | ||||
細胞培養液
餵養細胞系統 (Feeder-dependent)

The Standard Culture Medium for human ES/iPS cells
靈長類幹細胞與 iPS 細胞無血清培養基
Primate ES Cell MediumTM 是一個配方完整可以立即使用的產品,不含血清的培養基,專門為靈長類胚胎幹細胞生長增生所設計。使用前無須再添加任何添加物,且可使幹細胞維持正常型態、全能性及高度分化能力。山中伸彌教授於京都大學建立起全世界第一株 iPS 細胞便開始使用此款培養液,自 2005 年起,全球使用。
無餵養細胞系統 (Feeder-free)

ReproFF2: A New Feeder-Free Medium for human ES/iPS cells
新靈長類幹細胞與 iPS 細胞無血清、無餵養細胞培養基
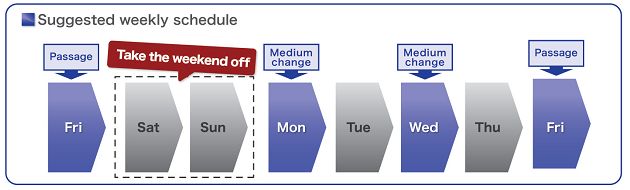
ReproFF2 TM 是一款無需血清及餵養細胞的培養基。不同於傳統的培養基需要每天更換, 使用 ReproFF2 每周 3 個工作天維護細胞。週一和周三的時候更換培養基,周五繼代細胞,因此,可節省培養基的花費、用量及人力時間。ReproFF2 只需預熱、加入 bFGF * 後即可使用。 (* 依細胞需求添加 bFGF)
Primate ES Cell Medium
請左右移動表格位置查看更完整內容
| 產品貨號 | 產品名稱 | 產品容量 |
|---|---|---|
| RCHEMD001 | Primate ES cell medium | 500mL |
| RCHEMD001A | Primate ES cell medium | 500mL x5 |
| RCHEMD001B | Primate ES cell medium | 500mL x5 & bFGF(25μL) pack |
ReproFF2
請左右移動表格位置查看更完整內容
| 產品貨號 | 產品名稱 | 產品容量 |
|---|---|---|
| RCHEMD006 | ReproFF2 | 500mL |
| RCHEMD006A | ReproFF2 | 500mL x3 |
| RCHEMD006B | ReproFF2 | 500mL x3 & bFGF(25μL) pack |
出版物
Primate ES Cell Medium
- Grajcarek J; Monlong J; Nishinaka-Arai Y; Nakamura M; Nagai M; Matsuo S; Lougheed D; Sakurai H; Saito MK; Borque G; Woltjen K. Genome-wide microhomologies enable precise template-free editing of biologically relevant deletion mutations. Nature Commun 10:4856 (2019).
- Takahashi M; Yamazaki S. Generation of a human induced pluripotent stem cell line, IMSUTi002-A-1, harboring the leukemia-specific fusion gene ETV6-RUNX1. Stem Cell Research 40: (2019).
- Takada H; Kaieda A; Tawada M; Nagino T: Sasa K; Oikawa T; Oki A; Sameshima T; Miyamoto K; Miytmoto M; Kokubu Y; Tozawa R; Sakurai H; Saito B. Identification of 2,6-Disubstituted-3H-imidazo[4,5-b]pyridines as Therapeutic Agents for Dysferlinopathies through Phenotypic Screening on Patient-Derived iPSCs. J Med Chem In press:1538 (2019).
- Umemura Y; Maki I; Tsuchiya Y; Koike N; Yagata K. Human circadian molecular oscillation development using induced pluripotent stem cells. J Biol Rhythms 34:525 (2019).
- Watanabe T; Yamazaki S; Yoneda N; Shinohara H; Tomioka I; Iiguchi T; Tagoto M; Ema M; Suemizu H; Kawai K; Sasaki E. Highly efficient induction of primate iPS cells by combining RNA transfection and chemical compounds. Genes to Cells in press:doi:10.1111/gtc.12702 (2019).
- Kuroda T; Yasuda S; Tachi S; Matsuyama S; Kusakawa S; Tano K; Miura T; Matsuyama A; Soto Y. SALL3 expression balance underlies lineage biases in human induced pluripotent stem cell differentiation. Nature Commun 10:2175 (2019).
- Minami T; Ishii T; Yasuchika K; Fukumitsu K; Ogiso S; Miyauchi Y; Kojima H; Kawai T; Ramaoka R; Oshima Y; Kawamoto H; Kotaka M; Yasuda K; Osufune K; Uemoto S. Novel hybrid three-dimensional artificial liver using human induced pluripotent stem cells and a rat decellularized liver scaffold. Regenerative Therapy 10:127 (2019).
- Ohashi F; Miyagawa S; Yasuda S; Miura T; Kuroda T; Itoh M; Kawaji H; Ito E; Yoshida S; Saito A; Sameshima T; Kawai J; Sawa Y; Sato Y. CXCL4/PF4 is a predictive biomarker of cardiac differentiation potential of human induced pluripotent stem cells. Sci Rep 9:3638 (2019).
- Nazeki F; Tsuge I; Horie T; Imamura K; Tsukita K; Hotta A; Baba O; Kuwabara Y; Nishino T; Nakao T; Nishiga M; Nishi H; Nakashima Y; Ide Y; Koyama S; Kimura M; Tsuji S; Maitoh M; Suzuki S; Izumi Y; Kawarai T; Kaji T; Kimura T; Inoue H; Ono K. MiR-33a is a therapeutic target in SPG4-related hereditary spastic paraplegia human neurons. Clinical Sci 133:583 (2019).
- Cieslar-Pobuda A; Rafat M; Knoflach V; Skonieczna M; Hudecki A; Malechi A; Urasinska E; Ghavami S; Los MJ. Human induced pluripotent stem cell differentiation and direct transdifferentiatoin into corneal epithelial-like cells. Oncotarget 7:42314 (2016).
- Morizane R; Lam AQ; Freedman BS; Kishi S; Valerius MT; Bonventre JV. Nephron organoids derived from human pluripotent stem cells model kidney development and injury. Nature Biotechnol 33:1193 (2015).
- Katayama S; Shimoda K; Yakenaga Y. Loss of ADAR1 in human iPS cells promotes caspase3‐mediated apoptotic cell death. Genes to Cells 20:675 (2015).
- Matsuura K, Wada M, Shimizu T, Haraguchi Y, Sato F, Sugiyama K, Konishi K, Shiba Y, Ichikawa H, Tachibana A, Ikeda U, Yamato M, Hagiwara N, Okano T. Creation of human cardiac cell sheets using pluripotent stem cells. Biochem Biophys Res Commun 425:321-7 (2012).
- Fukusumi H, Shofuda T, Bamba Y, Yamamoto A, Kanematsu D, Handa Y, Okita K,Nakamura M, Yamanaka S, Okano H, Kanemura Y. Establishment of Human Neural Progenitor Cells from Human Induced Pluripotent Stem Cells with Diverse Tissue Origins. Stem Cells Int. 2016;2016:7235757. doi: 10.1155/2016/7235757. Epub 2016 Apr 26. PubMed PMID: 27212953; PubMed Central PMCID: PMC4861799.
- D’Souza SS, Maufort J, Kumar A, Zhang J, Smuga-Otto K, Thomson JA, Slukvin II. GSK3β Inhibition Promotes Efficient Myeloid and Lymphoid Hematopoiesis from Non-human Primate-Induced Pluripotent Stem Cells. Stem Cell Reports. 2016 Feb 9;6(2):243-56. doi: 10.1016/j.stemcr.2015.12.010. Epub 2016 Jan 21. PubMed PMID: 26805448.
- Araoka, Toshikazu, et al. “Efficient and Rapid Induction of Human iPSCs/ESCs into Nephrogenic Intermediate Mesoderm Using Small Molecule-Based Differentiation Methods.” PloS one 9.1 (2014): e84881.
- Diederichs, Solvig, and Rocky S. Tuan. “Functional Comparison of Human Induced Pluripotent Stem Cell-Derived Mesenchymal Cells and Bone Marrow-Derived Mesenchymal Stromal Cells from the Same Donor.” Stem cells and development ja (2014).
- Hirata, Nao, et al. “A Chemical Probe that Labels Human Pluripotent Stem Cells.” Cell reports 6.6 (2014): 1165-1174.
- Kanemura, Hoshimi, et al. “Tumorigenicity Studies of Induced Pluripotent Stem Cell (iPSC)-Derived Retinal Pigment Epithelium (RPE) for the Treatment of Age-Related Macular Degeneration.” PloS one 9.1 (2014): e85336.
- Kimbrel, Erin A., et al. “Mesenchymal stem cell population derived from human pluripotent stem cells displays potent immunomodulatory and therapeutic properties.” Stem cells and development ja (2014).
- Koh, Sehwon, and Jorge A. Piedrahita. “From “ES-like” cells to induced pluripotent stem cells: A historical perspective in domestic animals.” Theriogenology 81.1 (2014): 103-111.
- Morishima, Tatsuya, et al. “Genetic correction of HAX1 in induced pluripotent stem cells from a patient with severe congenital neutropenia improves defective granulopoiesis.” Haematologica 99.1 (2014): 19-27.
- Takahashi, Daisuke, et al. “Electroporation of Adherent Cells by Direct Lamination of Hydrogel-Based Microelectrode Substrates.” Chemistry Letters 43.4 (2014): 444-446.
- Fukusumi, Hayato, et al. “Feeder-free generation and long-term culture of human induced pluripotent stem cells using pericellular matrix of decidua derived mesenchymal cells.” PloS one 8.1 (2013): e55226.
- Haraguchi, Yuji, et al. “Simple suspension culture system of human iPS cells maintaining their pluripotency for cardiac cell sheet engineering.” Journal of tissue engineering and regenerative medicine (2013).
- Hayakawa, Kazuo, et al. “Identification of target genes of synovial sarcoma-associated fusion oncoprotein using human pluripotent stem cells.” Biochemical and biophysical research communications 432.4 (2013): 713-719.
- Hitomi, Toshiaki, et al. “Downregulation of Securin by the variant RNF213 R4810K (rs112735431, G> A) reduces angiogenic activity of induced pluripotent stem cell-derived vascular endothelial cells from moyamoya patients.” Biochemical and biophysical research communications 438.1 (2013): 13-19.
- Hongisto, Heidi. “Fibroblast feeder cells in human pluripotent stem cell culture and retinal differentiation-progress toward clinical cell therapy.” (2013).
- Horii, Takuro, et al. “Generation of an ICF syndrome model by efficient genome editing of human induced pluripotent stem cells using the CRISPR system.” International journal of molecular sciences 14.10 (2013): 19774-19781.
- Iida, K., et al. “hypoxia-enhanced Derivation of ipscs from human Dental pulp cells.” Journal of dental research 92.10 (2013): 905-910.
- Lan, Chen-Wei, et al. “Differentiation of human embryonic stem cells into functional ovarian granulosa-like cells.” The Journal of Clinical Endocrinology & Metabolism 98.9 (2013): 3713-3723.
- Maeda, Tadao, et al. “Retinal pigmented epithelial cells obtained from human induced pluripotent stem cells possess functional visual cycle enzymes in vitro and in vivo.” Journal of Biological Chemistry 288.48 (2013): 34484-34493.
- Matsumoto, Yoshihisa, et al. “Induced pluripotent stem cells from patients with human fibrodysplasia ossificans progressiva show increased mineralization and cartilage formation.” Orphanet journal of rare diseases 8.1 (2013): 190.
- Nasu, Akira, et al. “Genetically matched human iPS cells reveal that propensity for cartilage and bone differentiation differs with clones, not cell type of origin.” PloS one 8.1 (2013): e53771.
- Noguchi, Michio, et al. “In vitro characterization and engraftment of adipocytes derived from human induced pluripotent stem cells and embryonic stem cells.” Stem cells and development 22.21 (2013): 2895-2905.
- Okita, Keisuke, et al. “An Efficient Nonviral Method to Generate Integration‐Free Human‐Induced Pluripotent Stem Cells from Cord Blood and Peripheral Blood Cells.” Stem Cells 31.3 (2013): 458-466.
- Sakuma, Tetsushi, et al. “Efficient TALEN construction and evaluation methods for human cell and animal applications.” Genes to Cells 18.4 (2013): 315-326.(Primate か Stem かは不明)
- Takase, Osamu, et al. “The Role of NF-κB Signaling in the Maintenance of Pluripotency of Human Induced Pluripotent Stem Cells.” PloS one 8.2 (2013): e56399.
- Tanabe, Koji, et al. “INAUGURAL ARTICLE by a Recently Elected Academy Member: Maturation, not initiation, is the major roadblock during reprogramming toward pluripotency from human fibroblasts.” Proceedings of the National Academy of Sciences of the United States of America 110.30 (2013): 12172.
- Tanabe, Koji, et al. “Maturation, not initiation, is the major roadblock during reprogramming toward pluripotency from human fibroblasts.” Proceedings of the National Academy of Sciences 110.30 (2013): 12172-12179.
- Tanaka, Akihito, et al. “Efficient and reproducible myogenic differentiation from human iPS cells: prospects for modeling Miyoshi Myopathy in vitro.” PloS one 8.4 (2013): e61540.
- Fujikura, J., et al. “Induced pluripotent stem cells generated from diabetic patients with mitochondrial DNA A3243G mutation.” Diabetologia 55.6 (2012): 1689-1698.
- Jung, Dongju, et al. “Incorporation of functionalized gold nanoparticles into nanofibers for enhanced attachment and differentiation of mammalian cells.” Journal of nanobiotechnology 10.1 (2012): 1-10.
- Kajiwara, Masatoshi, et al. “Donor-dependent variations in hepatic differentiation from human-induced pluripotent stem cells.” Proceedings of the National Academy of Sciences 109.31 (2012): 12538-12543.
- Kunisada, Yuya, et al. “Small molecules induce efficient differentiation into insulin-producing cells from human induced pluripotent stem cells.” Stem cell research 8.2 (2012): 274-284.
- Kuroda, Takuya, et al. “Highly sensitive in vitro methods for detection of residual undifferentiated cells in retinal pigment epithelial cells derived from human iPS cells.” PloS one 7.5 (2012): e37342.
- Matsuura, Katsuhisa, et al. “Creation of human cardiac cell sheets using pluripotent stem cells.” Biochemical and biophysical research communications 425.2 (2012): 321-327.
- Nakahata, Tatsutoshi, et al. “Method for producing dendritic cells from pluripotent stem cells.” U.S. Patent Application 14/001,004.
- Nakamura, Naoko, et al. “Feeder-free and serum-free production of hepatocytes, cholangiocytes, and their proliferating progenitors from human pluripotent stem cells: application to liver-specific functional and cytotoxic assays.” Cellular Reprogramming (Formerly” Cloning and Stem Cells”) 14.2 (2012): 171-185.
- Okahara-Narita, Junko, et al. “Induction of pluripotent stem cells from fetal and adult cynomolgus monkey fibroblasts using four human transcription factors.” Primates 53.2 (2012): 205-213.
- Shinozawa, Tadahiro, et al. “A novel purification method of murine embryonic stem cell?and human-induced pluripotent stem cell?derived cardiomyocytes by simple manual dissociation.” Journal of biomolecular screening 17.5 (2012): 683-691.
- Shinozawa, Tadahiro, et al. “Determination of Appropriate Stage of Human-Induced Pluripotent Stem Cell?Derived Cardiomyocytes for Drug Screening and Pharmacological Evaluation In Vitro.” Journal of biomolecular screening 17.9 (2012): 1192-1203.
- Sonoyama, Takuhiro, et al. “Differentiation of human embryonic stem cells and human induced pluripotent stem cells into steroid-producing cells.” Endocrinology 153.9 (2012): 4336-4345.
- Tanaka, Takayuki, et al. “Induced pluripotent stem cells from CINCA syndrome patients as a model for dissecting somatic mosaicism and drug discovery.” Blood 120.6 (2012): 1299-1308.
- Wada, Tamaki, et al. “Amyotrophic lateral sclerosis model derived from human embryonic stem cells overexpressing mutant superoxide dismutase 1.” Stem cells translational medicine 1.5 (2012): 396-402.
- Wakao, Shohei, et al. “Morphologic and gene expression criteria for identifying human induced pluripotent stem cells.” PloS one 7.12 (2012): e48677.
- Wutz, Anton. “Epigenetic alterations in human pluripotent stem cells: a tale of two cultures.” Cell stem cell 11.1 (2012): 9-15.
- Iwabuchi, Kumiko A., et al. “ECAT11/L1td1 Is Enriched in ESCs and Rapidly Activated During iPSCGeneration, but It Is Dispensable for the Maintenance and Induction of Pluripotency.” PloS one 6.5 (2011): e20461.
- Kobayashi, Hideyuki. “Pluripotent Stem Cells Induced from Testicular Tissue of a Man with Klinefelter Syndrome (47, XXY) by Four Transcription Factors (OCT4, SOX2, KLF4, and C-MYC).” (2011).
- Maekawa, Momoko, et al. “Direct reprogramming of somatic cells is promoted by maternal transcription factor Glis1.” Nature 474.7350 (2011): 225-229.
- Ogawa, Shin-ichiro, et al. “Induction of oligodendrocyte differentiation from adult human fibroblast-derived induced pluripotent stem cells.” In Vitro Cellular & Developmental Biology-Animal 47.7 (2011): 464-469.
- Okita, Keisuke, et al. “A more efficient method to generate integration-free human iPS cells.” Nature methods 8.5 (2011): 409-412.
- Takata, Akemi, et al. “Direct differentiation of hepatic cells from human induced pluripotent stem cells using a limited number of cytokines.” Hepatology international 5.4 (2011): 890-898.
- Tatsumi, Rie, et al. “Simple and highly efficient method for production of endothelial cells from human embryonic stem cells.” Cell transplantation 20.9 (2011): 1423-1430.
- Yahata, Naoki, et al. “Anti-Aβ drug screening platform using human iPS cell-derived neurons for the treatment of Alzheimer’s disease.” PLoS One 6.9 (2011): e25788.
- Fujioka, Tsuyoshi, et al. “Establishment of induced pluripotent stem cells from human neonatal tissues.” Human cell 23.3 (2010): 113-118.
- Kazuki, Yasuhiro, et al. “Complete genetic correction of ips cells from Duchenne muscular dystrophy.” Molecular Therapy 18.2 (2010): 386-393.
- Lee, Tae-Hee, et al. “Functional recapitulation of smooth muscle cells via induced pluripotent stem cells from human aortic smooth muscle cells.” Circulation research 106.1 (2010): 120-128.
- Miyoshi, Keiko, et al. “Generation of human induced pluripotent stem cells from oral mucosa.” Journal of bioscience and bioengineering 110.3 (2010): 345-350.
- Otsuji, Tomomi G., et al. “Progressive maturation in contracting cardiomyocytes derived from human embryonic stem cells: Qualitative effects on electrophysiological responses to drugs.” Stem cell research 4.3 (2010): 201-213.
- Sakurai, Kenji, et al. “Efficient integration of transgenes into a defined locus in human embryonic stem cells.” Nucleic acids research 38.7 (2010): e96-e96.
- Tamaoki, N, et al. “Dental pulp cells for induced pluripotent stem cell banking.” Journal of dental research 89.8 (2010): 773-778.
- Oka, Y., et al. “293FT cells transduced with four transcription factors (OCT4, SOX2, NANOG, and LIN28) generate aberrant ES-like cells.” J Stem cell Regenerative Med 3 (2010): 149-56.
- Tamaoki, N., et al. “Dental pulp cells for induced pluripotent stem cell banking.”Journal of dental research 89.8 (2010): 773-778.
- Chen, Hsin-Fu, et al. “A reduced oxygen tension (5%) is not beneficial for maintaining human embryonic stem cells in the undifferentiated state with short splitting intervals.” Human Reproduction 24.1 (2009): 71-80.
- Hong, Hyenjong, et al. “Suppression of induced pluripotent stem cell generation by the p53?p21 pathway.” Nature 460.7259 (2009): 1132-1135.
- Nakahara, Masako, et al. “High-efficiency production of subculturable vascular endothelial cells from feeder-free human embryonic stem cells without cell-sorting technique.” Cloning and stem cells 11.4 (2009): 509-522.
- Ohnuki, Mari, Kazutoshi Takahashi, and Shinya Yamanaka. “Generation and characterization of human induced pluripotent stem cells.” Current protocols in stem cell biology (2009): 4A-2.
- Wada, Tamaki, et al. “Highly efficient differentiation and enrichment of spinal motor neurons derived from human and monkey embryonic stem cells.” PloS one 4.8 (2009): e6722.
- Fusaki, Noemi, et al. “Efficient induction of transgene-free human pluripotent stem cells using a vector based on Sendai virus, an RNA virus that does not integrate into the host genome.” Proceedings of the Japan Academy. Series B, Physical and biological sciences 85.8 (2008): 348-362.
- Takahashi, Kazutoshi, et al. “Induction of pluripotent stem cells from adult human fibroblasts by defined factors.” cell 131.5 (2007): 861-872.
ReproFF2
- Morizane R, et al. “Nephron organoids derived from human pluripotent stem cells model kidney development and injury.” Nat Biotechnol. 2015 Oct 12. doi: 10.1038/nbt.3392.
- Naoki Nishishita, et al. “Generation and Maintenance of iPSCs From CD34+Cord Blood Cells on Artificial Cell Attachment Substrate.” Biochemistry, Genetics and Molecular Biology, Chapter 5. http://dx.doi.org/10.5772/58591
- Kanemura, Hoshimi, et al. “Tumorigenicity Studies of Induced Pluripotent Stem Cell (iPSC)-Derived Retinal Pigment Epithelium (RPE) for the Treatment of Age-Related Macular Degeneration.” PloS one 9.1 (2014): e85336.
- Hasegawa, Tsuyoshi, et al. “Cancer‐associated fibroblasts might sustain the stemness of scirrhous gastric cancer cells via transforming growth factor‐β signaling.” International Journal of Cancer (2013).
- Kanemura, Hoshimi, et al. “Pigment epithelium-derived factor secreted from retinal pigment epithelium facilitates apoptotic cell death of iPSC.” Scientific reports 3 (2013).
- Lam, Albert Q., et al. “Rapid and Efficient Differentiation of Human Pluripotent Stem Cells into Intermediate Mesoderm That Forms Tubules Expressing Kidney Proximal Tubular Markers.” Journal of the American Society of Nephrology: JASN (2013).
- Murakami, Masashi, et al. “The use of granulocyte-colony stimulating factor induced mobilization for isolation of dental pulp stem cells with high regenerative potential.” Biomaterials 34.36 (2013): 9036-9047.
- Nishishita, Naoki, et al. “Generation of virus-free induced pluripotent stem cell clones on a synthetic matrix via a single cell subcloning in the naive state.” PloS one 7.6 (2012): e38389.
- Sugiyama, Norikazu, et al. “Label-free characterization of living human induced pluripotent stem cells by subcellular topographic imaging technique using full-field quantitative phase microscopy coupled with interference reflection microscopy.” Biomedical optics express 3.9 (2012): 2175-2183.

