無餵養系統 (Feeder free) 培養 hESC /iPSC 最佳塗佈液!!!
-
適用於其他無餵養細胞的細胞培養液 (feeder-free media)
-
廣泛應用在幹細胞培養的相關研究,例如建立 iPSC
-
適用於重新編程 iPS 細胞的過程
-
每組批號的產品皆經過 iPSC 培養測試
使用方式
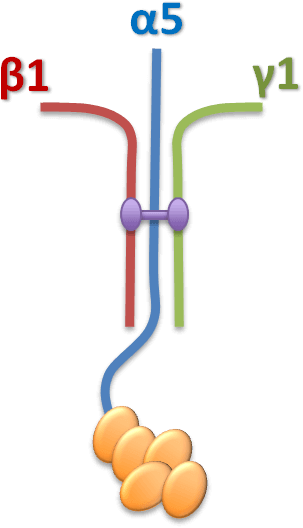
STEP1 Coating
- 以 PBS 稀釋 iMatrix-511
- 以 05 ug/cm2 塗佈細胞培養盤
STEP2 Seeding
- 移除多餘的塗佈液
- 直接將細胞培養在未乾燥的培養盤上
培養實例
請左右移動表格位置查看更完整內容
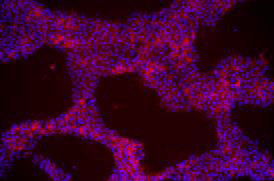
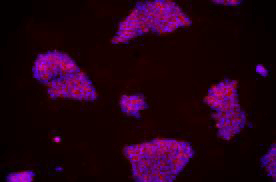
| A) Fibroblasts | B) EPCs | C) UDCs |
|---|---|---|
 |
 |
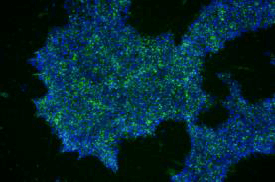 |
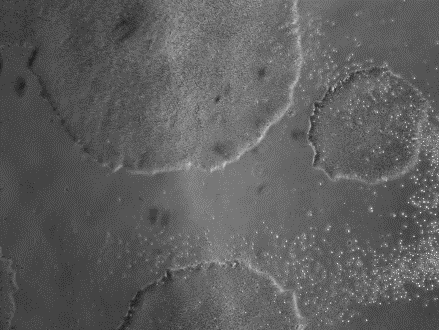
由不同種類細胞重新編程為 iPSC 於 iMatrix 上培養。使用螢光染劑標定幹細胞 (Stemgent StainAlive TRA-1-60 Antibody, Cat. No.09-0068)
| A) NutriStem | B) StemFit |
|---|---|
 |
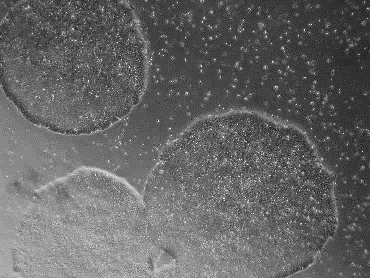 |
使用不同無餵養細胞的細胞液,皆適用 iMatrix 來成功培養 iPSC
Stem Cell Culture Substrate
請左右移動表格位置查看更完整內容
| 產品貨號 | 產品名稱 | 產品描述 | 產品容量 |
|---|---|---|---|
| NP892-011 | iMatrix-511 | highly purified and refined laminin-511 E8 fragments, produced in CHO cells | 175 ug × 2 tube/box |
| NP892-012 | 175 ug × 6 tube/box | ||
| NP892-013 | 175 ug × 1 tube/box | ||
| NP892-021 | iMatrix-511 SILK | xeno-free, recombinant laminin-511 E8 fragments, produced in recombinant silkworm cocoon | 175 ug × 6 tube/box |
| NP892-022 | 175 ug × 1 tube/box |
Endothelial Cell Substrate
請左右移動表格位置查看更完整內容
| 產品貨號 | 產品名稱 | 產品描述 | 產品容量 |
|---|---|---|---|
| NP892-041 | iMatrix 411 | highly purified and refined product of human recombinant laminin-411 (E8 fragment) expressed by CHO-S cells. | 175 ug × 2 tube/box |
| NP892-042 | 175 ug × 6 tube/box | ||
| NP892-043 | 175 ug × 1 tube/box |
Cardiac and Myoblast Cell Culture Substrate
請左右移動表格位置查看更完整內容
| 產品貨號 | 產品名稱 | 產品描述 | 產品容量 |
|---|---|---|---|
| NP892-061 | iMatrix 211 | highly purified and refined product of human recombinant laminin-221 (E8 fragment) expressed by CHO-S cells. | 175 ug × 2 tube/box |
| NP892-062 | 175 ug × 6 tube/box | ||
| NP892-063 | 175 ug × 1 tube/box |
- Takayama K. et al., “Laminin 411 and 511 promote the cholangiocyte differentiation of human induced pluripotent stem cells”. Biochemical and Biophysical Research Commun.474 (1): 91-96 (2016).
- Nishimura K. et al., “Estradial facilitates functional integration of iPSC-derived dopaminergic neurons into striatal neuronal circuits via activation of integrin a5b1”. Stem Cell Reports6 (4): 511-524 (2016).
- Matsuno K. et al., “Redefining definitive endoderm subtypes by robust induction of human induced pluripotent stem cells”. Differentiation2016.04.002.
- Hayashi R. et al., “Co-ordinated ocular development ofrom human iPS cells and recovery of corneal function”. Nature531, 368-80 (2016),
- Sasaki K. et al., “Robust in vitro induction of human germ cell fate from pluripotent stem cells”. Cell Stem Cell 17 (2):178-194 (2015).
- Okumura N. et al., “Laminin-511 and -521 enable efficient in vitro expansion of human corneal endothelial cells”. Invest Ophthalmal Vis Sci.56 (5), 2933-42 (2015).
- Nakagawa M. et al., “A novel efficient feeder-free culture system for the derivation of human induced pluripotent stem cells”. Scientific Reports 4: 3594 (2014).
- Miyazaki T. et al. “Laminin E8 fragments support efficient adhesion and expansion of dissociated human pluripotent stem cells.” Nature Communications 3: 1236 (2012).
- Taniguchi Y. et al., “The C-terminal region of laminin β-chains modulates the integrin-binding affinities of laminins.” J. Biol. Chem.284 (12): 7820-31 (2009).
- Ido H. et al., “The requirement of the glutamic acid residue at the third position from the carboxyl termini of the laminin gamma-chains in integrin-binding by laminins.” J. Biol. Chem.282 (15): 11144-54 (2017).
Additional Publications
- Shimada M; Tsukada K; Kagawa N; Matsumoto Y. Reprogramming and differentiation-dependent transcriptional alteration of DNA damage response and apoptosis genes in human induced pluripotent stem cells. J Radiation Res 2019:1-10 (2019).
- Nakajima M; Yoshimatsu S; Sato T; Nakamura M; Okahara J; Sasaki E; Shiozawa S; Okano H. Establishment of induced pluripotent stem cells from common marmoset fibroblasts by RNA-based reprogramming. Biochem Biophys Research Commun in press:https://doi.org/10.1016/j.bbrc.2019.05.175 (2019).
- Liu L-P; Li Y-M; Guo N-N; LI S; Ma X; Zhang Y-X; Gao Y; Huang J-L; Zheng D-X; Wang L-Y; Xu H; Hui L; Zheng Y-W. Therapeutic Potential of Patient iPSC-Derived iMelanocytes in Autologous Transplantation. Cell Reports 27:455-466.e5 (2019).
- Harmanto Y; Maki T; Yakagi Y; Miyamoto S; Takahashi J. Xeno‐free culture for generation of forebrain oligodendrocyte precursor cells from human pluripotent stem cells. J Neuro Res 2019:1-18 (2019).
- Tsujimara T; Takase O; Yoshikawa M; Sano E; Hayashi M; Hoshi K; Takato T; Toyoda A; Okano H; Hishikawa K. Controlling gene activation by enhancers through a drug-inducible topological insulator. bioRxiv http://dx.doi.org/10.1101/534073 (2019).


